Human Research Ethics Applications @ JCU Singapore
Human Research Ethics Applications @ JCU Singapore
IMPORTANT INFORMATION
All members of the research team on a HREC application must complete two mandatory training components:
These training documents must be submitted as part of the GECO HREC application.
APPLYING FOR HREC APPROVAL USING GECO
All HREC applications must be made via GECO from January 1, 2025.
You can find GECO here: https://www.jcu.edu.au/research-and-innovation-services/geco
When logging in use the JCU SSO option NOT email
CHOOSING THE RIGHT REVIEW PROCESS
The review process depends on the level of risk for your study according to the National Statement Section 2 Chapter 2.1 Risk and Benefit.
Exempt
When using GECO, on the Project Information tab, Ethics Review Details section you indicate that you are applying for an exemption. The screenshot below shows the correct section and response.

Lower Risk
If you are a JCU Singapore researcher and are applying for a Low Risk application, please follow two important steps. If you do not follow these steps, the timely review of your application may be effected.
STEP 1:
In the Collaborators and Funding tab, Ethics Advisor section you DO NOT need to select an Ethics Advisor. You can leave this section blank. The screenshot below shows the correct section and response.
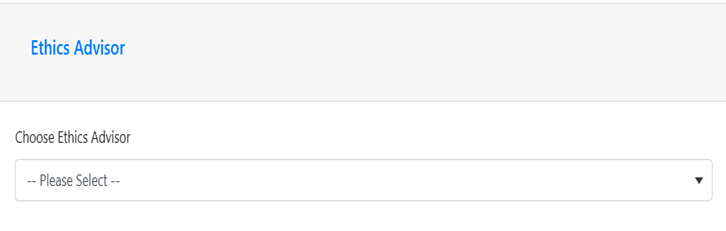
STEP 2:
When using GECO, on the Risk tab, Section F. Risk Category section select the Lower risk research box. This will direct your HREC application to the Lower Risk Executive Review Panel - Singapore.

Higher Risk
In the Collaborators and Funding tab, Ethics Advisor you mustselect an Ethics Advisor to review your HREC application.
In the Risk tab, Section F. Risk Category select the Higher risk research category.
For more information about Human Research Ethics, please click HERE.
NEED HELP?
Singapore: Lower Risk Panel Lead, Associate Professor Maria Hennessy at maria.hennessy@jcu.edu.au
Australia: ethics@.jcu.edu.au
SONA Research Participation Program
https://www.jcu.edu.sg/research/research-participation-program